Registering adverse reactions helps generate a database of evidence.
Anyone suffering from persistent benzodiazepine or Z-drug adverse drug reactions is encouraged to report them to the appropriate agency for this purpose.
IF YOU RESIDE IN THE UNITED STATES
Report to FDA’S MEDWATCH: (click below for detailed instructions)
Please note the FDA’s Privacy and Confidentiality information in regards to the MedWatch Voluntary Report.
Who should report to the FDA’s MedWatch Program?
- Injured patients (the ones who took benzodiazepines as prescribed) who are located in the United States
- Family members (if the patient taking the benzodiazepine died – e.g., from suicide or CT)
- Your doctor who is aware of your BZ adverse event/injury (you will have to ask them to do it on your behalf, as most don’t self-report even though they are supposed to)
How to fill out the MedWatch Online Voluntary Reporting Form:
The form may be accessed at:
https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home
Important points:
- This form is relatively easy to fill out and self-explanatory but detailed instructions are provided below
- There are 4 pages that need to be filled out online (page 1,3,4,5). Please push “next” after completing a page to move on to the next one (located at bottom of page)
- Page 6 is to review your information and edit if needed. If everything looks good, click on “submit” and you are done!
- Please report on benzodiazepines or Z-drugs only
- Items with a red star by them are required
- Please provide as much detailed information as possible
Page 1
Click on Consumer/Patient 
2. About the problem
 What kind of problem was it?
Check the first box (were hurt or had a bad side effect)
What kind of problem was it?
Check the first box (were hurt or had a bad side effect)
Did any of the following happen? Check all that apply including hospitalization, disability or health problem, life-threatening, and/or death
Date of occurrence Use approximate date you first noted problems related to your medication
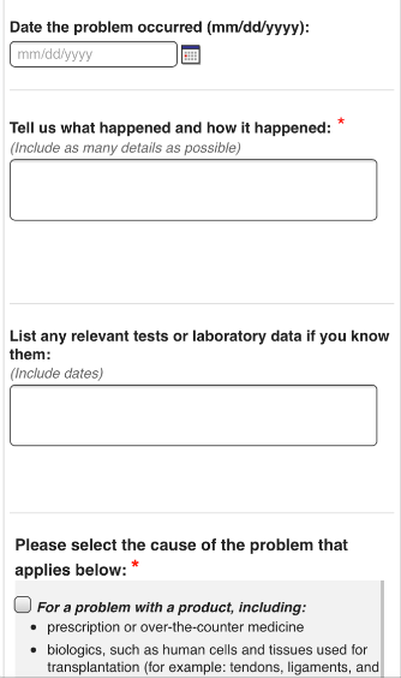
Tell us what happened and how it happened:
Include all relevant details such as:
- Benzodiazepine drug you were taking and dose, please note if you took it as prescribed
- How long you were on the drug
- Method of discontinuation (long taper, rapid taper, cold turkey, detox)
- Reason you were started on the drug
- List all symptoms you noticed while taking or discontinuing the drug (make sure to include severe symptoms such as suicidal ideation, attempted suicide, seizures, profound disability, bedridden, etc)
- The length of time you have been experiencing symptoms
- Any hospitalizations related to the drug or withdrawal process
- Death related to the drug
Avoid emotional language and stick to the facts.
List any relevant tests or laboratory data (include normal results to show that symptoms were not due to some other process), examples include:
- Brain MRI
- Lumbar puncture
- Electromyelogram/nerve conduction study
- Formal cognitive testing
- Cardiac testing
- Chest X-ray or CT
- Routine blood work
- Anything else you had done that proves disability from the BZ drug or withdrawal

Please select the cause of the problem that applies below:
- Check the first box (for a problem with a product)
- Do you still have the product? Check yes or no
Page 3
This page has been filled in for example purposes only. Please provide your own answers.

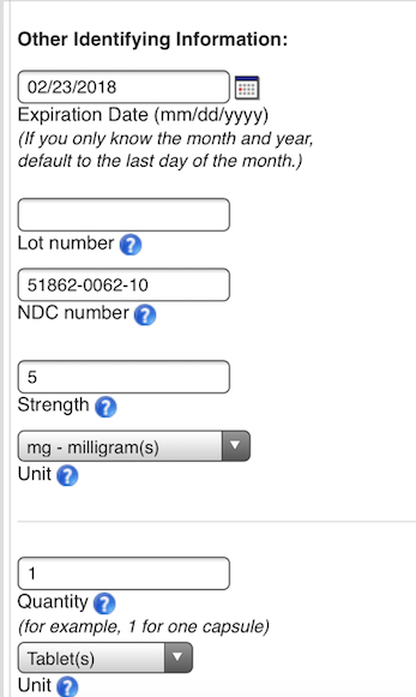
Click the blue question mark if you need more detail about the question.
Lot number can be obtained from your pharmacy but is not necessary. NDC number is on your prescription label. You do not need to enter these numbers if currently off the medication (unless you still have a prescription bottle).
If not currently on benzos, enter the maximum dose you were on previously.

If you took more than one benzo, click on add another product.
Page 4



Enter patient information.
Page 5


Add reporter (your) information. Check the yellow box at the bottom if you do not want your identity disclosed to the manufacturer.
Page 6
Review and edit your information. Press submit when you are done.
IF YOU RESIDE IN THE UK
Report to the Yellow Card Scheme
Click on “side effects”
Select whether you are a member of the public or a healthcare professional (you can ask your healthcare professionals to complete this reporting as well)
Then go on to complete the form, providing as much information as possible about your benzodiazepine/Z-drug adverse experience.
IF YOU RESIDE IN CANADA
Report to HEALTH CANADA
Complete all mandatory fields, marked by a *, and provide as much detail as possible for the remaining fields.
IF YOU RESIDE IN AUSTRALIA
Report to
IF YOU RESIDE IN NEW ZEALAND
Report to Center for Adverse Reactions Monitoring (CARM)
FOR ALL OTHER COUNTRIES
See the World Health Organization’s Collaborating Centre for International Drug Monitoring list of pharmacovigilance agencies. (click on the map to find the agency which represents your location)
TO MAKE YOUR VOICE HEARD
Report at RxISK.org
In addition to agencies like the U.S. Food and Drug Administration and World Health Organization, patients are encouraged to report their persistent side effects to RxISK.org, a global, independent pharmacovigilance organization whose database is used by patients, doctors and pharmacists to research prescription drugs and their side effects, ideally to identify problems early on in the life of a drug.